Category: hypoactive sexual desire dysfunction HSDD

Jean Hailes – Sex & Relationships
Menopause can affect your relationships and your sex life. Symptoms such as a dry vagina can make sex painful and you may find you… Continue Reading →

AMS Fact Sheet – Menopause before 40 and spontaneous premature ovarian insufficiency
Menopause before 40 and spontaneous premature ovarian insufficiency (POI) MAIN POINTS Premature ovarian insufficiency (POI) is a loss of function of the ovaries in… Continue Reading →
AMS Fact Sheet – Will menopause affect my sex life?
Will menopause affect my sex life? MAIN POINTS If your sex life is good before menopause, it is likely to remain good after menopause…. Continue Reading →
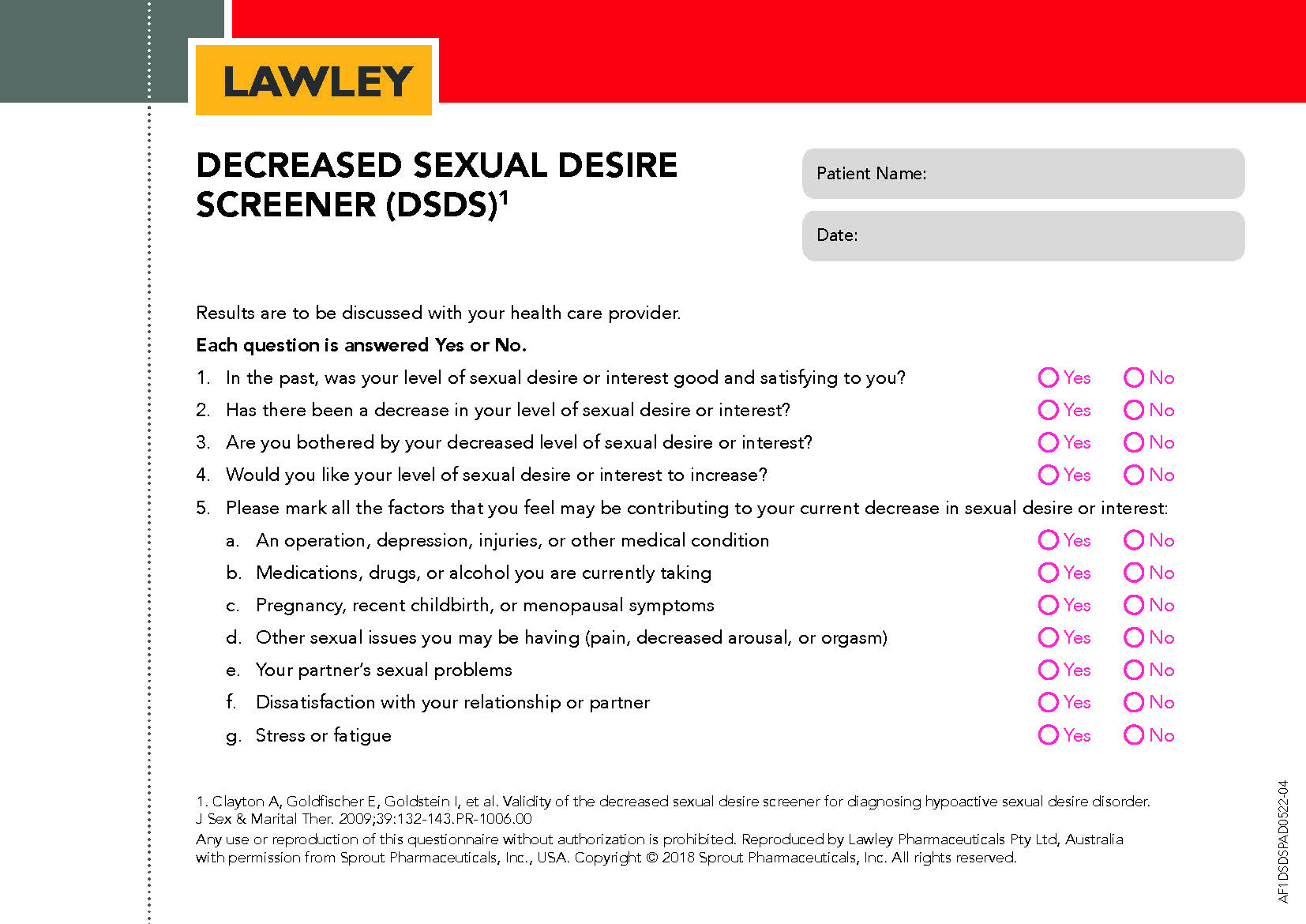
Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Low Sexual Desire is Common Sexual difficulties and concerns are common across a woman’s lifespan, increasing at midlife and beyond menopause. The DSDS (Decreased… Continue Reading →

Every Menopause Journey is Unique – Low Sexual Desire Is Common
Low Sexual Desire Is Common 1 in 3 women between the ages 40 – 64 will experience HSDD* which can severely impair relationships, mental… Continue Reading →
It’s time to #BreakTheBias around menopause.
If we just understood menopause a bit better, maybe we could #BreakTheBias Menopause is a big issue. Yet how many people really understand it,… Continue Reading →
Oprah Winfrey suggests menopause is a moment for women to reinvent themselves
Menopause is having a moment. While often historically seen as a ‘taboo’ women’s health issue, according to SATC star Kim Cattrall, menopause is becoming… Continue Reading →

Jean Hailes: Jean Hailes Magazine Vol. 1, 2021: Sex, anti-inflammatory foods, PMDD
From go to ‘ohh!’: Sex across the lifespan Through the course of a lifetime, a person’s sex life will ebb and flow. It can… Continue Reading →

Jean Hailes: Sex & Menopause
Sex & Menopause Menopause can affect your relationships and your sex life. Symptoms such as a dry vagina can make sex painful and you… Continue Reading →
Podcast: SexTok with Zibby and Tracey
This weekly show pairs two mismatched women discussing relationship and sex topics we all typically whisper about. Laugh-out-loud funny, irreverent, British, international sex expert… Continue Reading →





