TGA accepts AndroFeme 1 for evaluation in postmenopausal women with Hypoactive Sexual Desire Dysfunction.

TGA accepts AndroFeme 1 for evaluation in postmenopausal women with HSDD.
Perth, Western Australia; Dec. 05, 2019 — Lawley Pharmaceuticals today announced AndroFeme 1, an innovative formulation of testosterone cream for use in postmenopausal women with hypoactive sexual desire dysfunction (HSDD), has been accepted for evaluation by the Australian Therapeutic Goods Administration (TGA).
“The acceptance of the AndroFeme 1 dossier is the next step in women gaining access to a regulated safe and effective therapy that is female specific” said Michael Buckley, Lawley’s Medical Director. “AndroFeme 1 addresses an unmet clinical need for postmenopausal women who experience hypoactive sexual desire dysfunction (HSDD). Australian researchers lead the world in this area of menopausal medicine and TGA approval will open the way for an Australian company to supply the world with a product that global menopause experts and their patients desperately want.”
Testosterone is the primary sex steroid produced by both men and women which influences sexual motivation. Men produce up to twenty times more testosterone than women. Testosterone is one of the most widely used androgen medications globally in men, however there are no testosterone formulations approved for use in women. For decades physicians have use male-approved testosterone formulations off-label in women or “bioidentical” formulations made by “compounding” pharmacies despite wide-spread condemnation by medical experts due to quality, safety and efficacy concerns. The recently released “Global Consensus Position Statement on the Use of Testosterone Therapy for Women” emphasised the “pressing need for more research into testosterone therapy for women and the development and licensing of products indicated specifically for women.” The Position Statement has been endorsed by The International Menopause Society, The Endocrine Society, The European Menopause and Andropause Society, The International Society for Sexual Medicine, The International Society for the Study of Women’s Sexual Health, The North American Menopause Society, The Federacion Latinoamericana de Sociedades de Climaterio y Menopausia, The Royal College of Obstetricians and Gynaecologists, The International Society of Endocrinology, The Endocrine Society of Australia, The Royal Australian and New Zealand College of Obstetricians and Gynaecologists and the Australasian Menopause Society.
Hypoactive sexual desire dysfunction (HSDD) affects one in three women (32.8%) aged between 40 and 64 years of age and is a major health issue for women worldwide.
HSSD is characterised by significant loss of sexual desire, diminished sexual motivation, and the absence of sexual pleasure, fantasy and arousal which creates personal distress. HSDD is often a significant stressor in partnered relationships; postmenopausal women are most affected.
Latest News
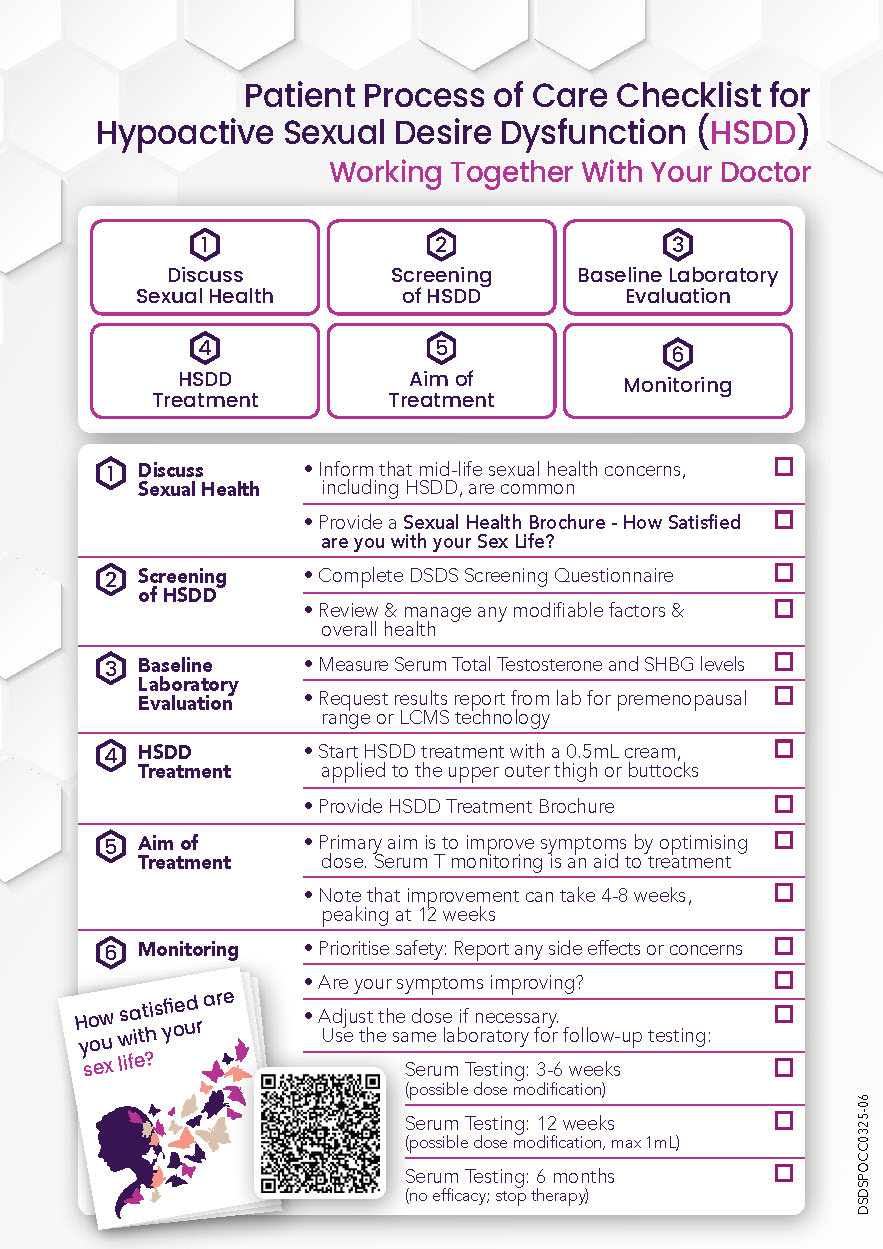
 HSDD – Patient Process of Care Checklist
HSDD – Patient Process of Care Checklist
Feeling Less Like Yourself? Let’s Talk About It. Sexual wellbeing is part of your overall health—and it’s okay to ask for help. If you’ve… Continue Reading →
 Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Low Sexual Desire is Common Sexual difficulties and concerns are common across a woman’s lifespan, increasing at midlife and beyond menopause. The DSDS (Decreased… Continue Reading →
 What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
International Society For Sexual Medicine What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?