INTERNATIONAL RECOGNITION PROCEDURE OFFERS HOPE FOR UK’S POSTMENOPAUSAL WOMEN.
Hypoactive Sexual Desire Dysfunction (HSDD)
Perth, Australia: Lawley Pharmaceuticals, via UK-based commercial partner, Andro Pharmaceuticals Ltd, today submitted a Marketing Authorisation Application (MAA) for AndroFeme cream (testosterone 10mg/ml) for women via the newly introduced International Recognition Procedure (IRP) for assessment by the UK regulator, the Medicines and Healthcare products Regulatory Agency (MHRA).
AndroFeme cream (testosterone 10mg/ml), a female specific testosterone formulation, has been licensed by Australian regulatory authorities since 2020 for the management of HSDD in postmenopausal women.
HSDD is characterised by the loss or absence of sexual desire associated with personal distress which affects between 6-13% of women in Europe. (Ref 1)
In March 2023 the AndroFeme dossier was validated for assessment under the MHRA national procedure, however has yet to progress in the queue due to a back-log of dossiers.
The MHRA IRP timetable for assessment via an IRP Recognition B pathway is 110-days.
When commenting on the 1 January 2024 launch of the IRP, Julian Beach, MHRA Interim Executive Director of Healthcare Quality and Access said:
“IRP allows us to access the expertise of trusted regulatory partners, who have already authorised products. In return, our partners can consider applications based on MHRA authorisations, creating a ‘win-win’ for regulators, developers of innovative treatments, and patients.”
Lawley’s Medical Director, Michael Buckley said “The IRP offers UK postmenopausal women renewed hope that they will soon be able to access to a female-specific formulation which will address the absence of a licensed testosterone treatment for HSDD”
About ANDROFEME Cream 10mg/ml (testosterone):
ANDROFEME cream (testosterone 10mg/ml) contains 1% w/v testosterone B.P (17-β- Hydroxyandrost-4-en-3-one).
This form of testosterone is identical to the testosterone produced by the ovaries and adrenal glands of women and the testes of men.
About Testosterone and HSDD:
Testosterone is the primary hormone that is vital for sexual motivation and desire in both sexes. Although it is commonly considered as a male hormone, testosterone is also produced by women in much smaller amounts.
A reduction in or an absence of sexual desire, thoughts, fantasies, responsiveness to erotic cues or stimulation is very common in menopausal and perimenopausal women over the age of 40 years. Medically any of the above symptoms that have persisted over several months or more with associated distress is classified as hypoactive sexual desire dysfunction (HSDD). Clinical studies have shown that testosterone therapy for postmenopausal women with HSDD, in doses that approximate physiological testosterone concentrations for premenopausal women, exert a beneficial effect on sexual function, that include increases in the subdomains of sexual desire, arousal, orgasmic function, pleasure and sexual responsiveness, together with a reduction in sexual concerns including sexual distress. (Ref 2)
About Lawley Pharmaceuticals Pty Ltd:
Lawley Pharmaceuticals Pty Ltd is an Australian company that specialises in the manufacture of pharmaceutical grade body-identical testosterone creams for the treatment of various endocrine deficiencies and related medical conditions.
Please direct all enquiries to Lawley Pharmaceuticals Pty Ltd by email to info@lawleypharm.com.au or via the website at www.lawleypharm.com.au
About Andro Pharmaceuticals Ltd:
Andro Pharmaceuticals Ltd is a new pharmaceutical company located in Guildford, UK focused on commercialising prescription medicines aimed at male and female endocrine healthcare.
End of Release.
References:
[1]. Nappi R et al. Management of hypoactive sexual desire disorder in women: current and emerging therapies. Int J Womens Health. 2010; 2: 167– 175.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2971736/
[2]. Davis SR et al. Global Consensus Position Statement on the Use of Testosterone Therapy for Women. J Clin Endo & Metabol 2019;104(10):4660-4666 https://academic.oup.com/jcem/article/104/10/4660/5556103
Latest News
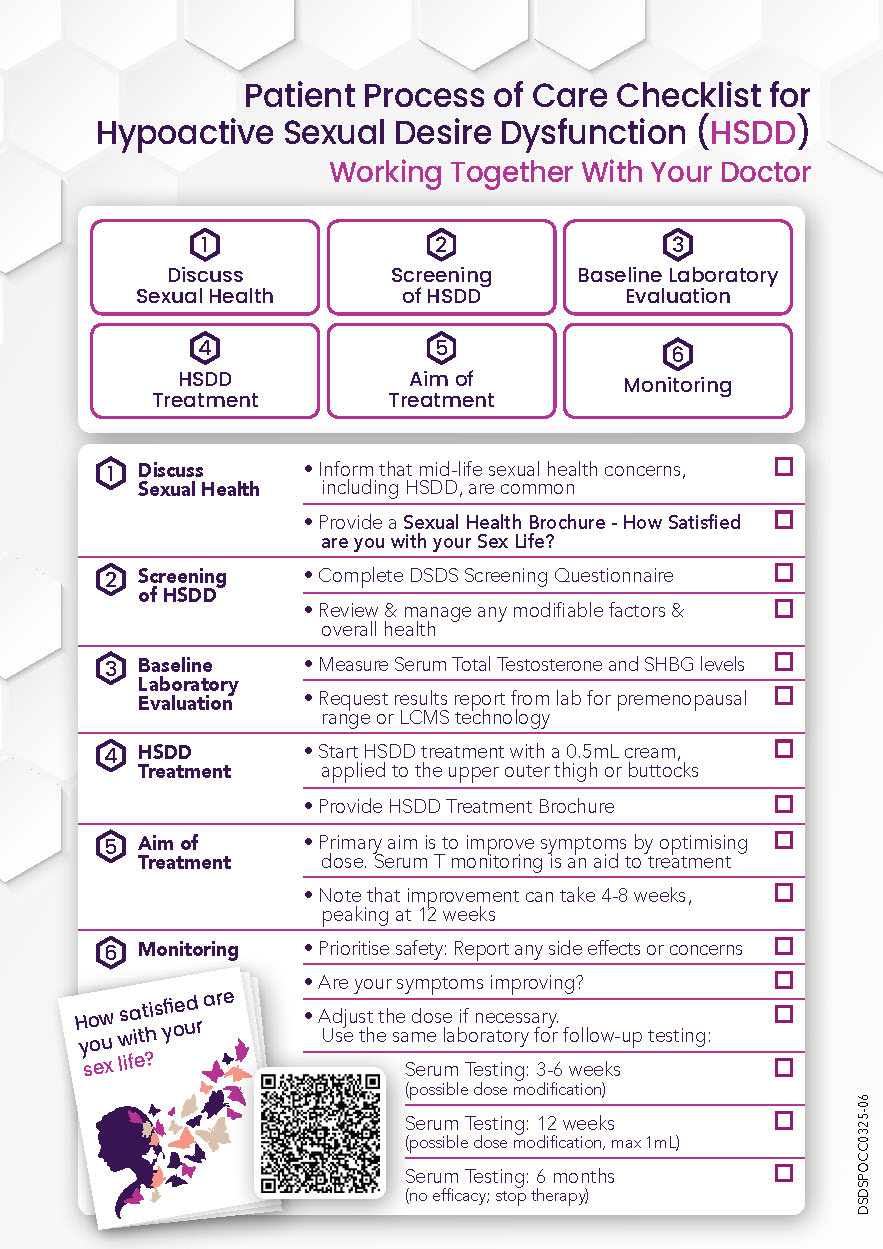
 HSDD – Patient Process of Care Checklist
HSDD – Patient Process of Care Checklist
Feeling Less Like Yourself? Let’s Talk About It. Sexual wellbeing is part of your overall health—and it’s okay to ask for help. If you’ve… Continue Reading →
 Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Low Sexual Desire is Common Sexual difficulties and concerns are common across a woman’s lifespan, increasing at midlife and beyond menopause. The DSDS (Decreased… Continue Reading →
 What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
International Society For Sexual Medicine What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?