Gender disparity in sexual health options for women: Experts call on pharma and drug regulators to address
In 2015 Comment in the prestigious medical journal Lancet Diabetes & Endocrinology by two of the world’s leading female sexual health specialists, Prof. Susan Davis (Australia) and Dr Sharon Parish (USA) stated:
“A well characterised testosterone formulation needs to be approved to protect women from inappropriate dosing when treated with testosterone formulated for men or in compounded form —which is nothing short of an unregulated human experiment.” 1
Last week another global KOL, Prof. Rosella Nappi from Italy, made a similar call in the same journal:
“In particular, there is an urgent need in the area of sexual medicine to ensure gender equality in treating effectively those women with female sexual dysfunction clearly related to hypoandrogenic states. However, products specifically approved in women should become available to achieve this goal” 2
This most recent plea follows the publication on 25th July 2019 of the most comprehensive systematic review and meta-analysis of testosterone treatment for women ever undertaken, involving 8,480 women. 3
The findings show the hormone testosterone significantly improves sexual wellbeing for postmenopausal women. Benefits include improved sexual desire, function and pleasure, together with reduced concerns and distress about sex.
Senior author Professor Susan Davis from Monash University, Australia said
“Nearly a third of women experience low sexual desire at midlife, with associated distress, but no approved testosterone formulation or product exists for them in any country and there are no internationally-agreed guidelines for testosterone use by women. Considering the benefits we found for women’s sex lives and personal wellbeing, new guidelines and new formulations are urgently needed.”
Although best known as a male hormone, testosterone is important for female sexual health, contributing to libido and orgasm as well as helping to maintain normal metabolic function, muscle strength, cognitive function and mood. Levels decline naturally over a woman’s lifespan, and can also drop sharply following surgical menopause. Prior research has suggested that testosterone therapy can improve sexual function in women, but the available formulations have been designed for men and evidence for their safety or for adverse side-effects in women is scant.
The authors reviewed the effects of treatments on sexual function and on measures of heart, cognitive and musculoskeletal health. The authors also looked for other serious side effects such as increased risk of heart disease or breast cancer, as well as the impact on mood and wellbeing, other measures of breast health such as mammographic density, metabolic effects, lipid profiles, and the development of androgenic effects such as increased hair growth.
This meta-analysis provides level 1 evidence that testosterone is safe and effective when used in appropriate doses in women experiencing diminished sexual motivation and distress.
References:
1. Davis SR and Parish SJ. Testosterone in women: can the challenges be met? Lancet Diabetes Endocrinol. 2015 Aug;3(8):588-90
2. Nappi RE. Testosteronefor women: green light for sex, amber light for health? Lancet Diabetes Endocrinol. 2019 Jul 25
3. Islam RM, Bell RJ, Green S, Page MJ, Davis SR. Safety and efficacy of testosterone for women: a systematic review and meta-analysis of randomised controlled trial data. Lancet Diabetes Endocrinol. 2019 Jul 25. https://www.thelancet.com/journals/landia/article/PIIS2213-8587(19)30189-5/fulltext
4. World Health Organisation ICD 11 HSDD https://icd.who.int/dev11/f/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1189253773
Latest News
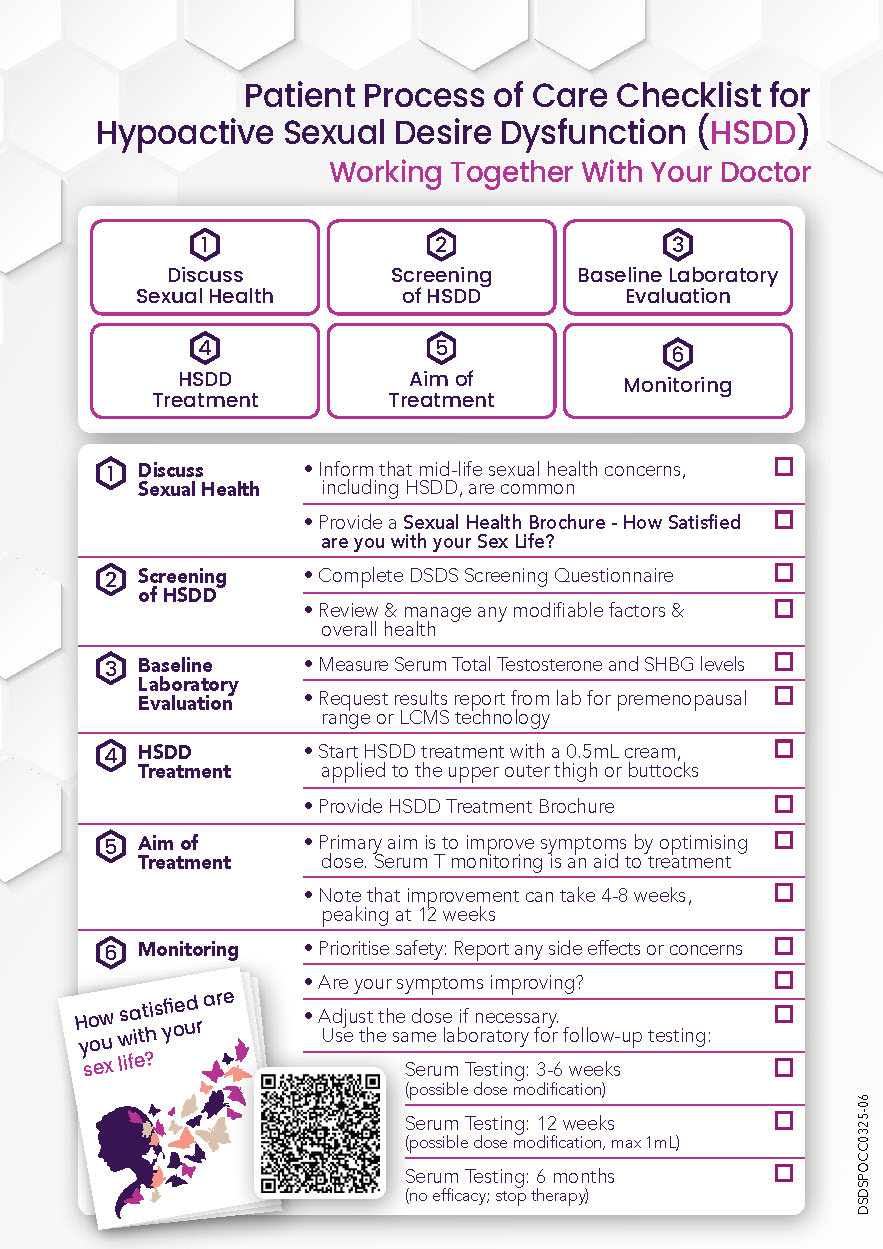
 HSDD – Patient Process of Care Checklist
HSDD – Patient Process of Care Checklist
Feeling Less Like Yourself? Let’s Talk About It. Sexual wellbeing is part of your overall health—and it’s okay to ask for help. If you’ve… Continue Reading →
 Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Low Sexual Desire is Common Sexual difficulties and concerns are common across a woman’s lifespan, increasing at midlife and beyond menopause. The DSDS (Decreased… Continue Reading →
 What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
International Society For Sexual Medicine What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?