FDA acknowledges there is a medical need for treating female sexual dysfunction
In late October 2016 the US FDA released draft guidance for Industry on Low Sexual Interest, Desire, and/or Arousal in Women: Developing Drugs for Treatment.
This document states ” Sexual dysfunction can adversely affect various aspects of life for a woman, including her relationship with her partner. There is a medical need for development of drugs with favourable benefit-risk profile to treat women with sexual dysfunction.”
This is a watershed publication with the world’s most stringent regulator acknowledging female sexual dysfunction is real and treatments are required.
Australia is unique in this area of female health, because unlike the rest of the world, Australian women and doctors have access to AndroFeme 1 testosterone cream form women.
AndroFeme 1 has proven clinical trial efficacy for low libido which addresses this important area of female sexual health.
Because the FDA have now clearly defined the regulatory pathway forward Lawley plans to undertake large scale clinical efficacy and safety studies to ultimately gain FDA and European regulatory approval.
To view the FDA document click here.
Latest News
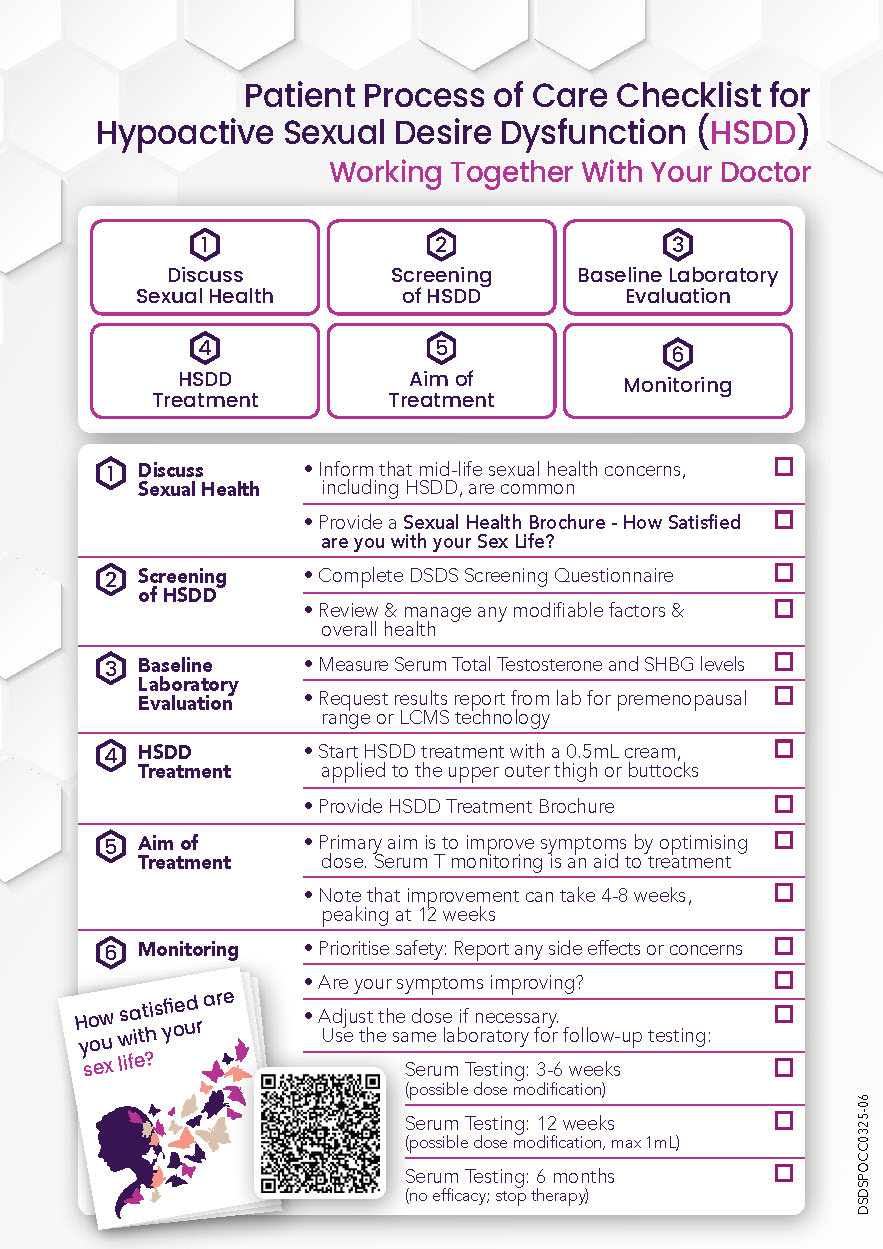
 HSDD – Patient Process of Care Checklist
HSDD – Patient Process of Care Checklist
Feeling Less Like Yourself? Let’s Talk About It. Sexual wellbeing is part of your overall health—and it’s okay to ask for help. If you’ve… Continue Reading →
 Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Low Sexual Desire is Common Sexual difficulties and concerns are common across a woman’s lifespan, increasing at midlife and beyond menopause. The DSDS (Decreased… Continue Reading →
 What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
International Society For Sexual Medicine What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?