AndroFeme 1 included on ARTG for hypoactive sexual desire dysfunction in postmenopausal women
Lawley Pharmaceuticals’ female-specific hormone cream, AndroFeme® 1, has been registered on the Australian Register of Therapeutic Goods (ARTG) for the treatment of postmenopausal women experiencing hypoactive sexual desire dysfunction (HSDD).
HSDD is characterised by low sexual desire with associated personal distress.
Australian research shows nearly one in every three Australian women aged 40 to 64 years experiences HSDD.
Registration on the ARTG means postmenopausal women now have access to a female-specific medically prescribed, clinically effective treatment which meets the requisite quality and safety standards for therapeutic use, as opposed to compounded formulations of off-label prescribed hormone products licensed for use in men.
Australian specialists and researchers have for decades pioneered ground-breaking research into female sexual dysfunction. This collective of work culminated in September 2019 with the release of the Global Consensus Position Statement on the Use of Testosterone Therapy for Women which was endorsed by almost 20 of the world’s leading medical societies and associations.
Women who experience signs or symptoms of HSDD should seek advice from their doctor.
AndroFeme® 1 is expected to be available in pharmacies nationally from April 2021.
Latest News
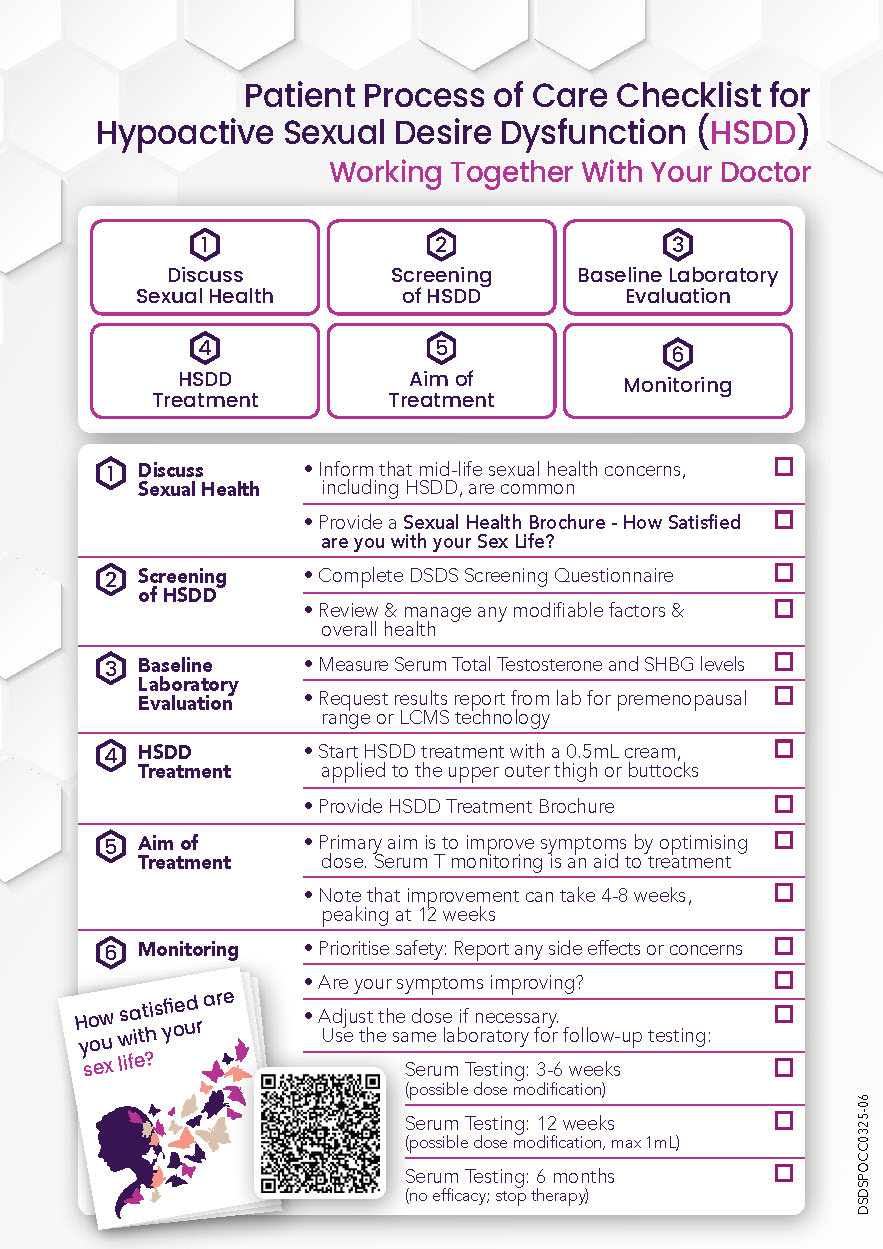
 HSDD – Patient Process of Care Checklist
HSDD – Patient Process of Care Checklist
Feeling Less Like Yourself? Let’s Talk About It. Sexual wellbeing is part of your overall health—and it’s okay to ask for help. If you’ve… Continue Reading →
 Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Did you know there is an initial screening tool to assist in the assessment of Hypoactive Sexual Desire Dysfunction (HSDD)?
Low Sexual Desire is Common Sexual difficulties and concerns are common across a woman’s lifespan, increasing at midlife and beyond menopause. The DSDS (Decreased… Continue Reading →
 What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?
International Society For Sexual Medicine What Is the Best Way to Approach a Sexual Dry Spell in a Relationship?