Participants Required For Men’s Testosterone Study
Professor David J Handelsman (Andrology Dept, Concord Hospital, ANZAC Research Institute, Sydney) is looking to recruit 15 healthy male volunteers to participate in a new testosterone study titled ‘Pharmacokinetics of Testosterone Cream Applied to Scrotal Skin’
The conventional use of testosterone cream, gel and solutions has been to the upper body in testosterone deficient men. Due to low absorption rates residual testosterone can be transferred to partners and children via contact with the skin to which the testosterone has been applied. This study will examine the absorption of testosterone cream when applied to scrotal skin which opens the potential to avoid passive transfer problems.
The study is seeking 15 men that meet the following criteria;
- healthy and aged between 18 and 50
- able to make 5 visits within a 12 day period, including 3 x 16 hour visits to the clinic
- have normal reproductive endocrine function.
If you are interested in participating please complete this screening questionnaire link www.lawleypharm.com.au/tstudy.php.
Participants will be compensated for their time and travel expenses at the end of participating in the study.
Latest News
 Sexual Function in Breast Cancer Survivors
Sexual Function in Breast Cancer Survivors
International Society For Sexual Medicine Sexual Function in Breast Cancer Survivors
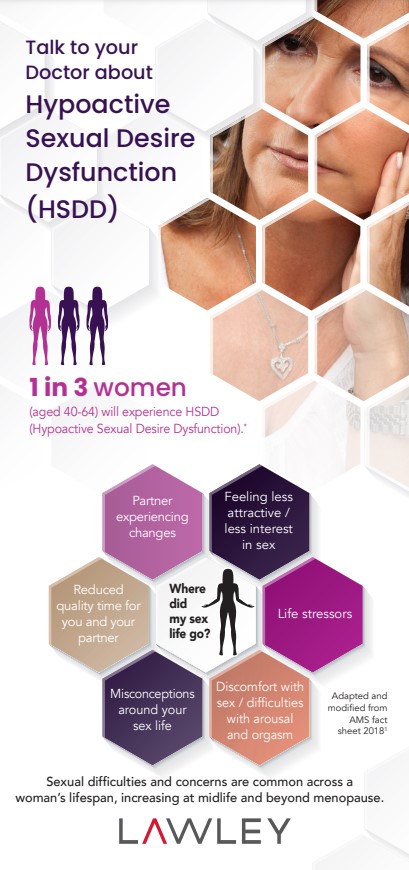
 How to talk to your Doctor about Sexual Health
How to talk to your Doctor about Sexual Health
What is HSDD*? *Hypoactive Sexual Desire Dysfunction Hypoactive sexual desire dysfunction (HSDD) is an extremely common medical condition that causes distress resulting from a… Continue Reading →